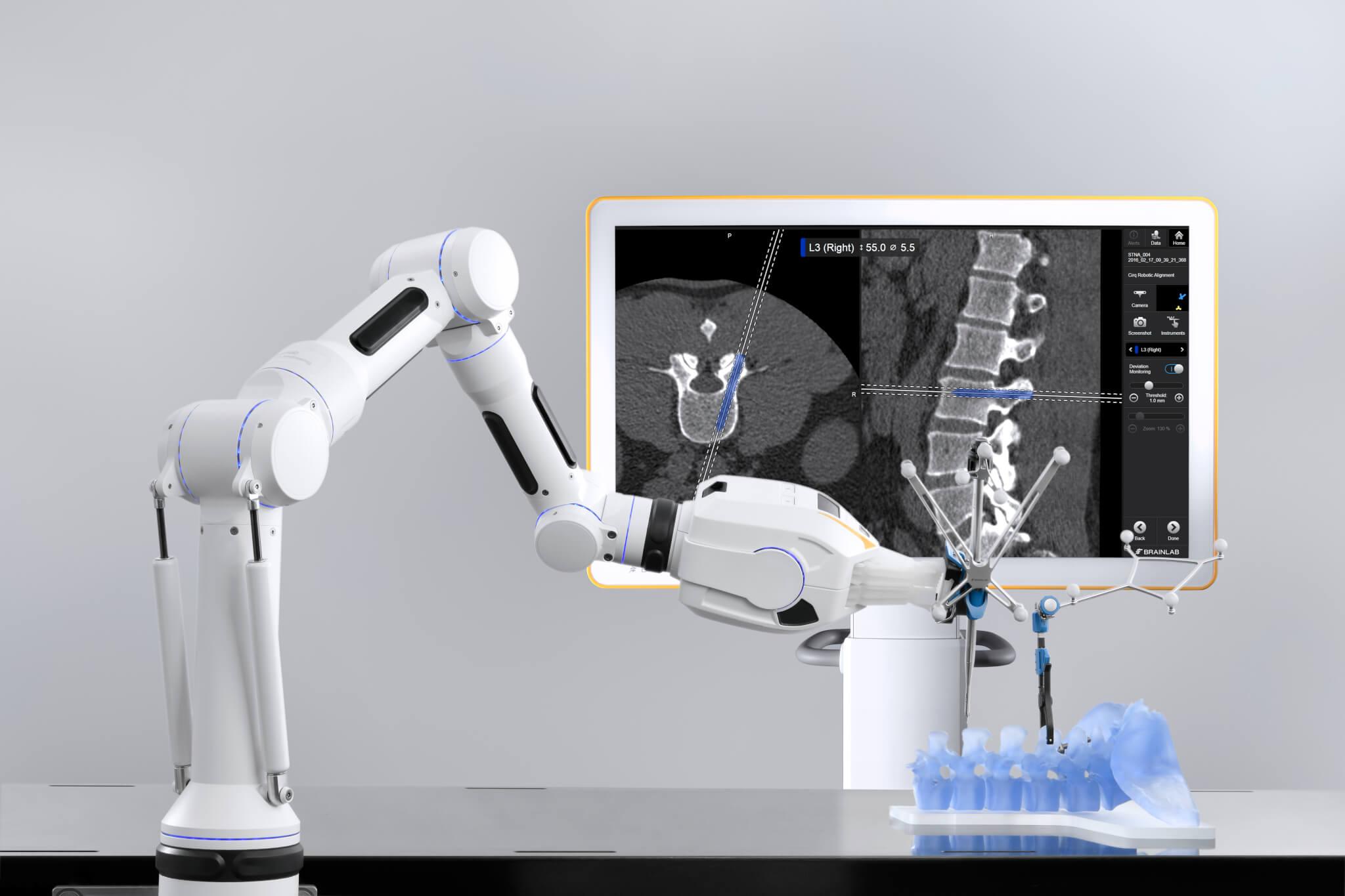
Advancing technology for clinicians and patients
Brainlab partners with clinical sites to conduct research with the aim of improving clinical outcomes, increasing patient satisfaction, and reducing hospitals’ costs.
Ways we support clinical studies and research
Clinical research can include any research conducted with human subjects at the clinical site. Scientific research includes animal, cadaveric, algorithm testing, and other research not involving living human subjects. Studies can either be sponsored by Brainlab or initiated by the investigator.
Brainlab sponsored studies
These studies are planned and sponsored by Brainlab with the clinical site conducting the research.
Brainlab roles & responsibilities:
Provide required Brainlab products and training
Draft the clinical cooperation agreement, including standard terms & conditions
Obtain necessary regulatory approval
Prepare the study protocol
Assure the validity of the study data
Principle investigator roles & responsibilities:
Check the data generated during the study
Conduct the study
Report safety data to regulatory authorities, IRB/EC and Brainlab
Joint submission to the IRB/EC
Submit study data updates in accordance with the clinical cooperation agreement
Ownership of intellectual property
Brainlab typically has rights to all intellectual property developed from Brainlab sponsored study data.
Data usage
Brainlab manages data and provides guidelines for subject consent.
Monitoring
Brainlab is responsible for organizing monitoring.
Investigator initiated studies
These studies are planned and sponsored by Brainlab with the clinical site conducting the research.
Brainlab roles & responsibilities:
Provide required Brainlab products and training
Draft the clinical cooperation agreement, including standard terms & conditions
Obtain necessary regulatory approval
Prepare the study protocol
Assure the validity of the study data
Principle investigator roles & responsibilities:
Check the data generated during the study
Conduct the study
Report safety data to regulatory authorities, IRB/EC and Brainlab
Joint submission to the IRB/EC
Submit study data updates in accordance with the clinical cooperation agreement
Ownership of intellectual property
Brainlab typically has rights to all intellectual property developed from Brainlab sponsored study data.
Data usage
Brainlab manages data and provides guidelines for subject consent.
Monitoring
Brainlab is responsible for organizing monitoring.
Reimbursement
If support is being requested in the form of reimbursement, both Brainlab and the investigator must agree on a budget plan. The budget includes a list of deliverables from both parties and costs must reflect fair market value.
Provision of products
Brainlab may provide support through the provision of products for the duration of the clinical cooperation. If provided, we will offer appropriate training and technical support of those provided products.
Support with study documents
We offer our support with completing study documents, such as EC/IRB approval, manuscripts for publication, study protocols, white papers, case report forms and patient consent, as indicated in the clinical cooperation agreement.
How to start a clinical study
Are you interested in beginning research with Brainlab? Click below to fill out the clinical study request form and we’ll review your proposal.
Clinical Cooperation Process
What happens during the clinical cooperation process?
Submit Request
Submit a request for a research project.
Request Review
Brainlab reviews the request and sends a reply. If your request is denied, you’ll receive an email.
Kick-Off
If your request is approved, we’ll reach out to set up a meeting to discuss the details.
Negotiate Contract
The clinical cooperation agreement and standard terms & conditions are negotiated.
Obtain IRB/EC Approval
Principal investigator obtains IRB/EC approval with Brainlab support, if necessary.
Initiate Study
Once all approvals are obtained, the study can begin.
Provide Products
Brainlab installs the products and trains the appropriate personnel.
Conduct Study
Principal investigator conducts the study and provides Brainlab with regular progress updates.
Close Study
Once all deliverables, as outlined in the clinical cooperation agreement, are received, the study is complete.
Become a clinical cooperation partner
We value the collection of post-market clinical data on our products and for that we rely on our valued cooperation partners. Interested in contributing? Select from one of the research topics we’re currently pursuing below.