Adaptive Hybrid Surgery Receives FDA Clearance
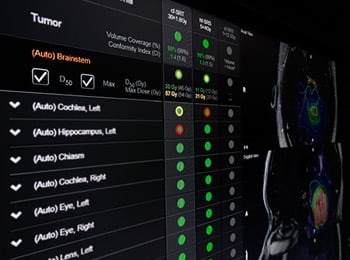
Brainlab announced 510(k) clearance for revolutionary software, Adaptive Hybrid Surgery. After successful clinical use in several international markets, Adaptive Hybrid Surgery is now available in the United States. Built with neurosurgeons in mind, Adaptive Hybrid Surgery helps physicians to balance surgical risk with radiosurgical toxicity for multi-disciplinary treatment of skull-base tumors. A complete set of automated tools enables continuous analysis of adjuvant radiosurgery at any time, while planning and performing a surgical resection. • On-the-fly feasibility analysis of adjuvant radio surgery • Complete treatment workflow from planning to intraoperative decision making • Support of interdisciplinary collaboration • Upfront integration of radiosurgery avoiding late referrals and potentially restricted treatment options Click here to learn more about Adaptive Hybrid Surgery.