Brainlab Acquires Robotics Platform Company Medineering
Brainlab creates first openly scalable medical robotics platform paired with an efficient software ecosystem
Brainlab, a global pioneer of software-driven medical technology, announced today the acquisition of Medineering, a developer of application-specific robotic technologies. With this move, Brainlab is driving the democratization of digital surgery with scalable solutions that expand clinical frontiers.
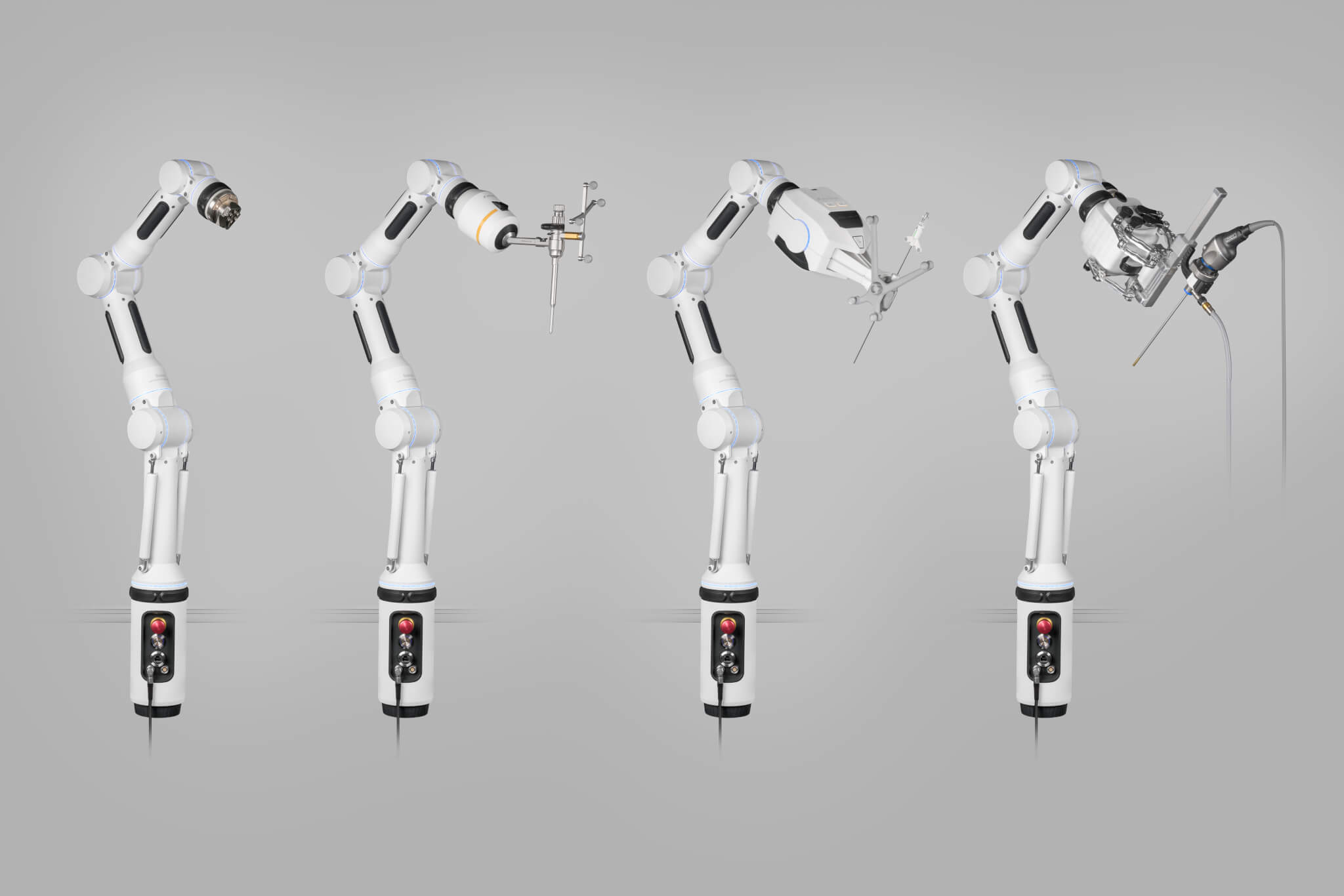
This strategic move increases the depth of the Brainlab portfolio in cranial surgery and strengthens its offering in spinal surgery, contributing a powerful digital step in clinical workflows. Brainlab currently markets the Medineering arm under the name Cirq®. Inspired by the form of the human arm, Cirq is an intuitive assistant during surgical procedures. After quick setup, highly adaptable Cirq can be aligned in seven degrees of freedom for maximum positioning flexibility. Once Cirq is locked firmly in place, surgeons are free to focus on subsequent surgical steps with both hands. Navigation integration leverages established workflows, set-up and instrumentation, and extends utilization. The combination of a base arm and attachable “hand” modules makes Cirq scalable and future-proof.
The acquisition adds another platform to the open hardware architecture of the Brainlab digital ecosystem for surgery, enabling other medical technology companies to design their own solutions and applications across many subspecialties. Unlike other closed monolithic industry offerings, this acquisition is expected to offer an open platform for a broad range of clinical opportunities.
Brainlab has been an investor and distribution partner for Medineering for nearly three years. In contrast to robotics concepts that start at over a million dollars, the light weight Medineering arm is available for a fraction of the cost and mounts easily to the siderails of the O.R. table, making the vendor-neutral robotic platform accessible to many of the over 4,000 existing Brainlab customers, and expanding market reach into ambulatory surgery centers. Medineering has demonstrated the feasibility of its mechatronic arm for holding a range of hands, from a straightforward instrument holder to robotic hands for the alignment of biopsy needles, drill guides, and endoscopes. Several applications are already available and in clinical use in Europe, under the name Cirq, with FDA clearance pending in the United States.
“Becoming part of Brainlab means scalability for our technology and improved market access,” comments Stephan Nowatschin, Co-Founder and CEO, Medineering. “Combining our open platform with the software ecosystem from Brainlab will enable more efficient development of very competitive clinical solutions.”
“Medineering introduced a fresh new approach to surgical robotics when we entered into our partnership less than three years ago,” said Stefan Vilsmeier, President and CEO, Brainlab. “Today, we are shifting gears and accelerating development with additional resources to address a broader clinical market.”
Brainlab
Brainlab creates software-driven med tech digitizing, automating and optimizing clinical workflows. Serving physicians, medical professionals and patients in 6700 hospitals in 127 countries, we’re transforming healthcare to improve the lives of patients everywhere. We employ over 2400 people in 25 locations worldwide. Visit and follow: Brainlab, LinkedIn, Twitter, Facebook and Instagram.
About Medineering
Medineering, headquartered in Munich, develops, manufactures and markets an application-specific and convenient robotic portfolio. The portfolio consists of the Medineering Positioning Arm and compact application-specific robots assisting surgeons in complex anatomical regions. Dr. Maximilian Krinninger and Dr. Stephan Nowatschin founded Medineering in June 2014. The startup has grown rapidly and the young and international team consists of highly specialized robotic engineers, marketing experts and regulatory specialists.